New Data on Protein Structure in Blood Cells
Emerging research on the 3D protein structure of a specific protein in red blood cells leads to greater understanding on how this and similarly shaped proteins work in the body.
By Emma Ryan
Every protein in the human body has a specific 3D shape that allows it to work properly and keep us healthy, including the ones we rely on to move carbon dioxide from our bodies to our breaths. For the first time, a research team from the Icahn School of Medicine at Mount Sinai has found the shape of one such protein, called anion exchanger 1 (AE1), with stunning precision. This study on protein structure allows us to better understand how proteins with similar shapes work and to develop new drugs for different blood diseases.
Protein structure: making blood thicker than water
The automatic ability of different proteins to form their specific shapes is a key mystery of biology. Imagine you wanted to make an origami bird: if it were like a protein, the paper could fold itself into the shape of a bird without needing your hands at all! Each incredibly diverse shape allows every protein to fulfill its specific biological role.
RELATED: Using a protein to reverse skeletal muscle aging
As you can guess, the cells in our blood also depend on the special shapes of proteins to carry nutrients and gasses throughout our bodies and protect us from disease and injury. A protein called hemoglobin in our red blood cells carries the oxygen we breathe in from our lungs all the way to our brain and toes. Hemoglobin’s structure—which is made up of a pair of two identical bunches of ribbon-like protein chains and iron atoms—was the first 3D protein structure to be identified.
When the oxygen carried by hemoglobin is used by our body to make energy, carbon dioxide is left over. If not brought to our lungs so we can exhale it out, carbon dioxide can quickly build up in the blood and become dangerous. One of the proteins on our red blood cells that moves carbon dioxide (in a form called bicarbonate) is called anion exchanger 1 (AE1). Although AE1 is the target of several important medicines, many of its structural details remained unclear. To uncover them, a group of researchers led by Dr. Daniel Wacker, Dr. Bin Zhang, and Dr. Avner Schlessinger at Mount Sinai investigated how human AE1 looks with never-before-seen detail.
Through the ice: the path to AE1
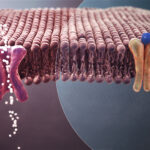
Dr. Wacker’s group used a technique called cryo-electron microscopy to see the protein structure of AE1 by itself and investigate whether it changed when bound by bicarbonate or different drugs. To do this, they froze AE1 at around -200ºC and used a powerful microscope to peer through the ice and capture many pictures of AE1 from different angles. With all these pictures, they recreated a 3D map of AE1.
This map showed that two identical protein chains come together to form AE1’s complete protein structure. One part of AE1, called the membrane domain, is anchored inside the red blood cell’s membrane, or the covering of the cell that separates its insides from the outside environment. Importantly, they also mapped certain features that decorate the face of the membrane domain that projects outside the cell. Called the Diego antigen system, these regions have been identified as the cause of several dangerous blood diseases in fetuses and newborn babies. If a mother’s immune system views the baby’s red blood cells as a threat at these sites, her antibodies will attack and destroy them.
For the first time, the other half of AE1 was shown to sit perpendicular to this membrane domain and protrude into the cell’s inside, or cytoplasm. Their work revealed that this region is incredibly flexible when unbound by drugs or bicarbonate and therefore does not have a specific, fixed structure like the membrane domain does.
A carbon dioxide elevator
The team’s reconstruction of the protein structure for AE1 also revealed the existence of a deep hole in the middle of the membrane domain, with a positively charged patch that allows AE1 to hold onto negatively charged bicarbonate. They were even able to pinpoint the exact atoms in AE1 that make this interaction possible!
This study gave further evidence for the idea that bicarbonate moves through the membrane domain via an elevator-like mechanism. After bicarbonate enters its binding site inside the membrane domain, the ribbon-like protein chains of AE1 that make up this site move downward to push bicarbonate into a pocket below the entry channel. From there, it enters the interior of the red blood cell at the lungs.
RELATED: “Rotten Egg” Gas’ Surprising Role in Breathing
By examining the protein structure of AE1 bound by the different drugs that target it, the team was able to describe different methods of inhibiting AE1’s elevator-like mechanism. The drug dipyridamole, a blood thinner that reduces clot formation, settles above the positively charged patch in the membrane domain and blocks bicarbonate’s access to this site. Another medicine, the painkiller niflumic acid, has an interesting method of stopping AE1. Rather than directly blocking the channel that bicarbonate travels through, niflumic acid prevents the downward movement of AE1’s protein chains.
New applications from AE1
Resolving the protein structure of human AE1 paves the way for broad and exciting new directions in human health and drug development. The full view of the Diego antigen system offered by this study allows for the development of new treatments that can protect from diseases that destroy red blood cells. Understanding how AE1 works to transport carbon dioxide and how different drugs prevent it from doing so can be applied toward other transport proteins in the body that look like AE1.
This study was published in the peer-reviewed journal Nature Structural & Molecular Biology.
References
Capper, M. J., Yang, S., Stone, A. C., Vatansever, S., Zilberg, G., Mathiharan, Y. K., Habib, R., Hutchinson, K., Zhao, Y., Schlessinger, A., Mezei, M., Osman, R., Zhang, B., & Wacker, D. (2023). Substrate binding and inhibition of the anion exchanger 1 transporter. Nature Structural & Molecular Biology, 30, 1495–1504. https://doi.org/10.1038/s41594-023-01085-6
Reithmeier, R. A. F., Casey, J. R., Kalli, A. C., Sansom, M. S. P., Alguel, Y., & Iwata, S. (2016). Band 3, the human red cell chloride/bicarbonate anion exchanger (AE1, SLC4A1), in a structural context. Biochimica et Biophysica Acta (BBA) – Biomembranes, 1858(7 Pt A), 1507–1532. https://doi.org/10.1016/j.bbamem.2016.03.030
Nelson, D. L., & Cox, M. M. (2017). Lehninger Principles of Biochemistry (7th ed.). W.H. Freeman.
About the Author
Emma Ryan is a post-baccalaureate research associate with the Geneva Foundation, conducting research into malaria vaccines and monoclonal antibody therapies. She is working to transition into a graduate education program that will enable her to develop structure-informed therapies for infectious diseases. In her free time, she enjoys cooking, fashion, and fish-keeping (her favorite fish is the betta/fighting fish!).