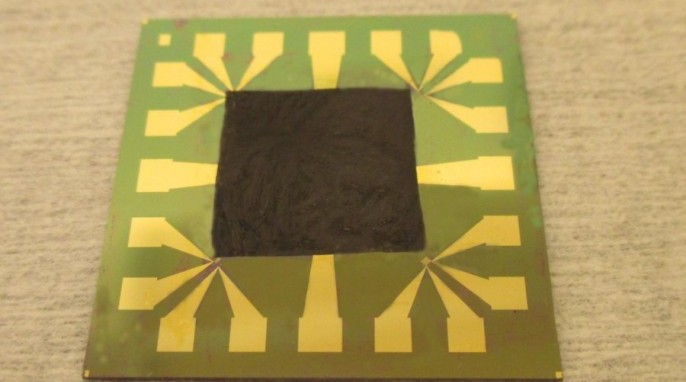
Scientists at Stanford University have just built a low-cost water splitter that uses a single catalyst to produce both hydrogen and oxygen gas. This is an invention that could pave the way for a boom in production of clean-burning cars.
The scientists say that their device, which can run nonstop for an unprecedented period of time, could provide a renewable source of clean-burning hydrogen fuel for transportation and industry. The team used lithium-ion battery technology to create one low-cost catalyst that is capable of driving the entire water-splitting reaction.

Clean Hydrogen Production
“We have developed a low-voltage, single-catalyst water splitter that continuously generates hydrogen and oxygen for more than 200 hours, an exciting world-record performance,” says Yi Cui, associate professor of materials science and engineering at Stanford and of photon science at the SLAC National Accelerator Laboratory.
Different research labs have been checking out a variety of alternative energy sources and previous EH Science reports on this topic include the following: a prototype that generates power from water evaporation, high-efficiency solar materials, and even a team taking alternative energy inspiration from giant clams. Now, cleaner and cheaper hydrogen is in play.
“Our group has pioneered the idea of using lithium-ion batteries to search for catalysts,” Cui continues. “Our hope is that this technique will lead to the discovery of new catalysts for other reactions beyond water splitting.”
How Clean is Clean Hydrogen?
Hydrogen has long been promoted as an emissions-free alternative to gasoline, but its use has not become widespread. Currently, most commercial-grade hydrogen is made from natural gas, a fossil fuel that contributes to global warming, so scientists have been trying to develop a cheap and efficient way to extract pure hydrogen from water.
A conventional water-splitting device consists of two electrodes submerged in a water-based electrolyte. A low-voltage current applied to the two electrodes drives a catalytic reaction that separates molecules of H2O, releasing bubbles of hydrogen on one electrode and oxygen on the other.
Each electrode is embedded with a different catalyst, typically platinum and iridium, which are two rare and costly metals. But in 2014, Stanford chemist Hongjie Dai developed a water splitter made of inexpensive nickel and iron that runs on an ordinary 1.5-volt battery.
Single Catalyst Clean Hydrogen Production
In pursuit of efficient and clean hydrogen production, Cui and his colleagues advanced Dai’s technology a step further. “Our water splitter is unique, because we only use one catalyst, nickel-iron oxide, for both electrodes,” explains graduate student Haotian Wang. “This bifunctional catalyst can split water continuously for more than a week with a steady input of just 1.5 volts of electricity. That’s an unprecedented water-splitting efficiency of 82 percent at room temperature.”
Wang and his colleagues discovered that nickel-iron oxide, which is cheap and easy to produce, is actually more stable than some commercial catalysts made of precious metals.
Batteries and Catalysis
To meet the two main challenges of cost and energy efficiency, the research team searched for catalytic material suitable for both electrodes. They borrowed a technique used in battery research called “lithium-induced electrochemical tuning” — a technique that uses lithium ions to chemically break the metal oxide catalyst into smaller and smaller pieces.
“Breaking down metal oxide into tiny particles increases its surface area and exposes lots of ultra-small, interconnected grain boundaries that become active sites for the water-splitting catalytic reaction,” Cui explains. “This process creates tiny particles that are strongly connected, so the catalyst has very good electrical conductivity and stability.”
Through a process of trial and error, they eventually discovered that nickel-iron oxide is a world-record performing material that can catalyze both the hydrogen and the oxygen reaction. “No other catalyst can do this with such great performance,” Cui says.
What is Next?
Using one catalyst made of nickel and iron significantly reduces the cost of producing hydrogen. It also opens up possibilities of other chemical fuels beyond hydrogen. “The technique has been used in battery research for many years, but it’s a new approach for catalysis. The marriage of these two fields is very powerful,” Cui says.
EH Science recently asked Cui when we might see this new technology in production for a mass market. He replied that more testing is needed before scaling up to an industrial scale water splitter. “Certainly, it might soon be used for mass production,” he tells us.
Funding for this research was provided by the Stanford University Global Climate and Energy Project and the Stanford Interdisciplinary Graduate Fellowship Program. These findings are published in the journal Nature Communications.
Video embedded with express permission from Stanford University. Photo Courtesy of L.A. Cicero/Stanford News Service.
GotScience.Org translates complex research findings into accessible insights on science, nature, and society. For the latest science news, follow on Twitter, Facebook, Google Plus, or subscribe to our science newsletter!