A taste-signaling protein found in the tongue and pancreas provides a way to design healthier sugar substitutes and new diabetes medications.
By Emery Haley
You take a bite of your favorite food and a soft “mmm” slips out as the complex layers of flavor dance across your tongue. Maybe it is the nice savory flavor of fried chicken, or the salty crunch of potato chips, or the sweet smoothness of chocolate ice cream. Whatever it is, you can thank your sense of taste.
But how do our taste buds relay those flavors to our brains? Recent work from my dissertation project explores the structure of TRPM5, an ion channel involved in transmitting signals from taste receptors. TRPM5’s structure holds promise not only for building our understanding of the molecular mechanisms of taste perception but also as a template for designing sugar substitutes and diabetes treatments.
“Taste” in the tongue and beyond
Our tongues are covered in little mound-shaped structures called taste buds. Within these taste buds are a collection of different taste-sensing cells, each with different receptor proteins. Type-II cells recognize bitter, sweet, and umami (savory) tastes, while type-III cells recognize sour tastes.
Once the taste receptors on these cells bind molecules from our food, a signal cascade is started that eventually transmits this taste signal to the brain. This signal transmission relies on ion channels, proteins that act like gates on the surface of cells, to let ions cross the cell membrane. The movement of these ions leads to the release of neurotransmitters, the molecules that neurons use to communicate information to, from, and within the brain.
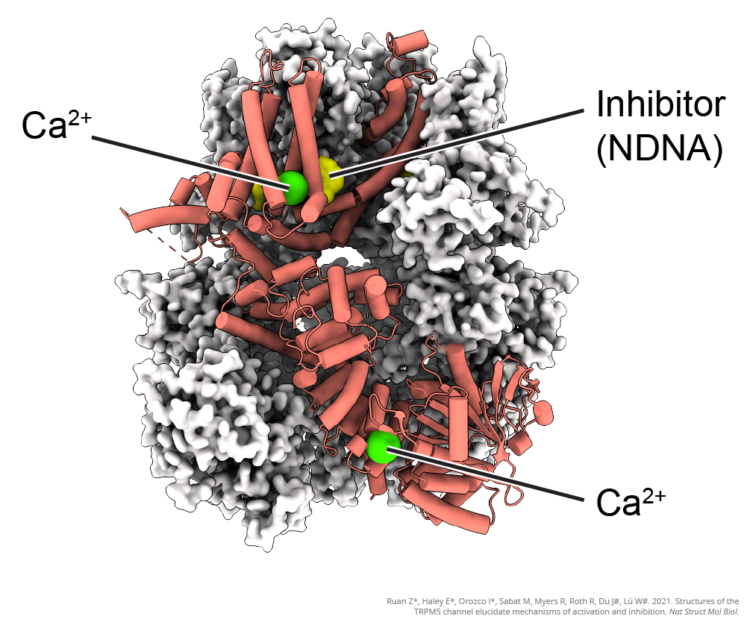
Surprisingly, taste-receptor proteins and the ion channels that help transmit their signals aren’t only found in taste-bud cells. These “taste” sensing functions are also important in other parts of the body, such as the intestinal tuft cells, where they use “taste” perception to recognize parasites and start immune responses. Another place where “taste” is important is the pancreas, where sugar molecules bind to taste receptors on β-islet cells to trigger insulin release.
One of the ion channels essential to transmitting “taste” signals is TRPM5. It is highly expressed in both type-II taste receptor cells and pancreatic β-islet cells. When activated, TRPM5 allows sodium ions to enter cells and depolarize the membrane. Membrane depolarization is a critical step in transmitting the taste signal. From mouse models, we know that TRPM5 activation is necessary for glucose-induced insulin release, and from human genetic studies, we know that TRPM5 expression is lower in patients with type-II diabetes. Because of this, we believe that increasing TRPM5 channel activation could boost insulin secretion in diabetic patients, a novel treatment strategy.
The molecular locksmithing of drug design
The goal of my work is to understand the structure of TRPM5. Understanding the structure is important because it will allow us to design a drug that specifically acts on the channel, much like the way a locksmith can design a perfectly fitting key only after learning what the inner structure of the lock is.
For my work, I use a specialized microscope called a cryo-electron microscope to capture 2D images of the TRPM5 ion channel. Because the channels are so tiny, each individual image is unclear, like when you try to take a picture with a camera phone using the maximum zoom. By taking millions of images of the channel from many different angles and using a computer to line them all up and average them together, we can get a clear image of the ion channel’s structure and build 3D models.
We can build models showing a channel in different states, such as “open” and “closed,” which allows us to see and understand how it works. We can also see how different drugs or molecules interact with the channel to cause it to open and close. With this understanding, we can design different drugs that fit into pockets on the channel like keys into a lock, allowing us to control the channel activity.
RELATED: New Study on Lowering Risk of Diabetes from Antipsychotic Drugs
Since TRPM5 is involved in the perception of sweetness as well as glucose-induced insulin release from the pancreas, molecules designed to interact with TRPM5 can serve multiple purposes. One potential use for such compounds is to treat metabolic disorders like type-II diabetes. In this case, molecules that can increase the activation of TRPM5 should increase insulin release in response to sugar consumption, helping to control blood sugar.
Another potential use is in sugar substitutes. Low-calorie or zero-calorie sweeteners are popular in soft drinks and other commercial food products today, especially for diabetics. However, existing sugar substitutes have had mixed results, with some being reported to paradoxically increase blood sugar. Since TRPM5 is located in both the tongue and pancreas, a molecule that activates TRPM5 would be sweet to the taste and also induce insulin release, just like sugar does naturally.
This study was published in the peer-reviewed journal Nature Structural & Molecular Biology.
RELATED: Giant Virus Evolution Key to New Medical Nanotechnology
References
Colsoul, B., Schraenen, A., Lemaire, K., Quintens, R., Van Lommel, L., Segal, A., Owsianik, G., Talavera, K., Voets, T., Margolskee, R. F., Kokrashvili, Z., Gilon, P., Nilius, B., Schuit, F. C., & Vennekens, R. (2010). Loss of high-frequency glucose-induced Ca2+ oscillations in pancreatic islets correlates with impaired glucose tolerance in Trpm5−/− mice. Proceedings of the National Academy of Sciences, USA, 107(11), 5208–5213.
Ruan, Z., Haley, E., Orozco, I. J., Sabat, M., Myers, R., Roth, R., Du, J., & Lü, W. (2021). Structures of the TRPM5 channel elucidate mechanisms of activation and inhibition. Nature Structural & Molecular Biology, 28, 604–613. https://doi.org/10.1038/s41594-021-00607-4
Tabur, S., Oztuzcu, S., Duzen, I. V., Ergün, S., & Ozkaya, M. (2015). Association between the transient receptor potential channel gene polymorphisms and metabolic syndrome. European Congress of Endocrinology, 37.

About the Author
Emery Haley is a nonbinary cell biologist with a passion for diversity in STEM. They are currently a PhD student at Van Andel Institute Graduate School and are planning to pursue a career in science communication. Connect with them on X (formerly Twitter) @EmeryHaley2.
More by this author
Why Don’t We Have Memories of Early Childhood?
Neuromodulation: How We Manipulate Brain Cells




