Biomedical engineers at Georgia Tech are designing a cellular tool to detect disease without the need for complex and costly lab equipment.
By William Harley
Nobody complains when things are easy. That is why we hear lots of frustration about healthcare. One main obstacle is arriving at a diagnosis. This is a critical and challenging first step toward managing disease. It enables individuals and communities to get the best treatment. However, the journey toward diagnosis is too often expensive and complicated since it often requires specialized equipment and trained professionals. This pushes many medical practices to even outsource their lab testing.
Consider how this impacts an individual from a rural community. First, they may need to drive hours to a doctor. After this great effort, they may need to send samples to a lab farther away to confirm a diagnosis. This poses major barriers toward people getting the care they need and has broader impacts in developing countries that might not even have the technology in their region.
Detecting disease with technology
A critical part of diagnosing disease is identifying biomarkers. Biomarkers are simply indicators of the disease in a patient and can be anything from foreign particles such as a virus or abnormal amounts of common human molecules. For example, cholesterol is an important molecule for many things in the human body including transporting fats, but a high amount of cholesterol would indicate heart disease.
There is a need for the early detection of disease to ensure early treatment and better health outcomes. Researchers are blazing their own path, circumventing the need for expensive equipment. They are designing simple diagnostics tools that can be sent directly to the individuals who need them. This is called Point-of-Care (POC) testing.
All of this talk of tools, equipment, and technology might suggest that the solution is yet another new machine with complicated buttons and a metallic exterior. But the new tool that researchers in Chemical and Biomolecular Engineering at Georgia Institute of Technology have developed is a liquid.
A cellular tool without the cell
To the human eye, this new tool is a liquid, while at the microscopic level, the researchers have harnessed the power of machinery found inside living creatures. All living creatures are made of cells that contain DNA—the instructions that define how an organism develops. These instructions have small sections called genes that code for unique building blocks and tools that perform a variety of roles in the cell, from keeping it alive to helping it duplicate—these are proteins. A special protein called a polymerase reads the DNA instructions and sends the messages to the protein. Using this fundamental biology that occurs in humans every day, the scientists designed a new cellular tool for detecting disease without the need for a cell.
The first step is creating part of the tool that recognizes biomarkers. The human body already has markers that recognize and label these markers as a part of the immune response. These are another form of proteins called antibodies. The part of the biomarker they bind to is called the antigen. Like a lock and key, there is only one antigen that matches an antibody. This portion of the protein that binds the marker, called a nanobody, is selected for the first part of the diagnostic tool.
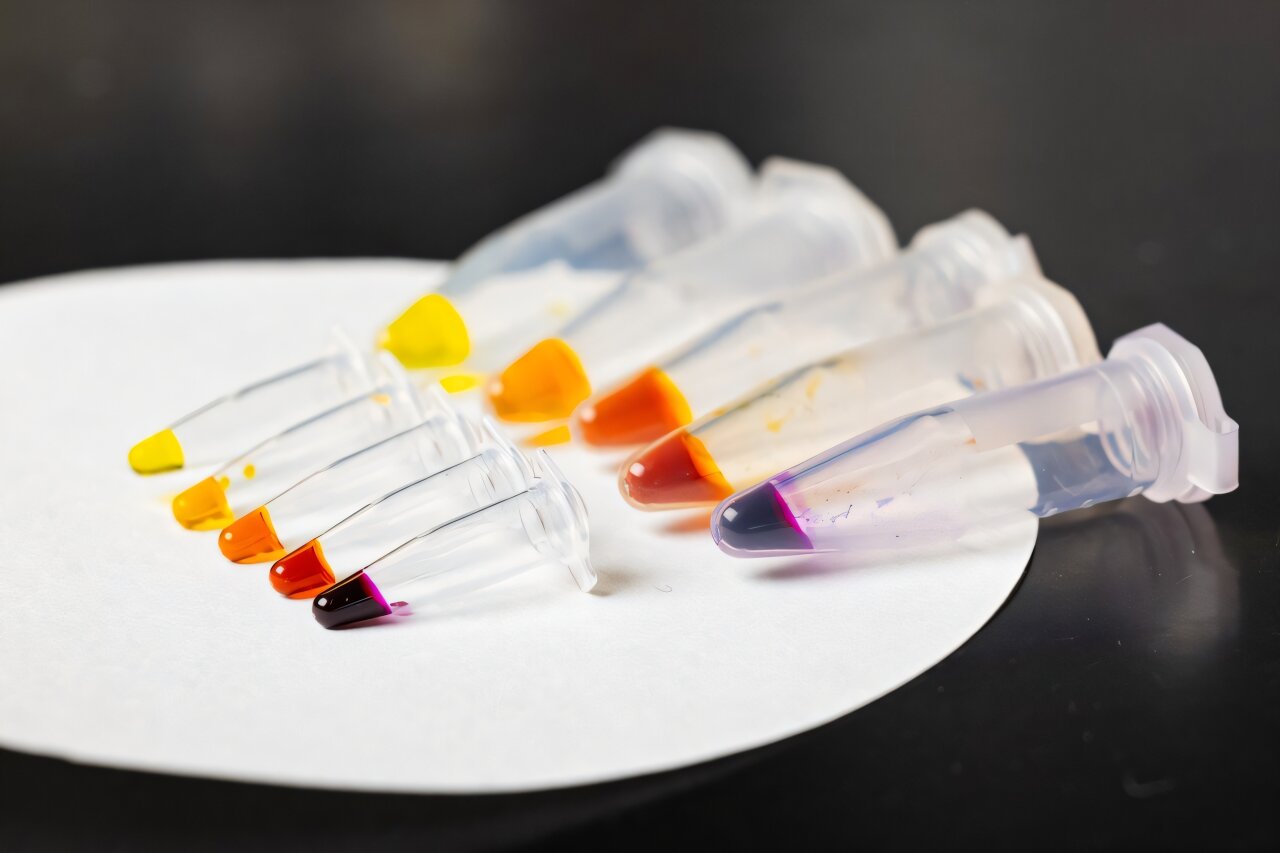
Second, a signal needs to be generated when the marker has been found. Instead of attaching a synthetic tool, the researchers continued to take inspiration from biology. They used the polymerase that reads DNA instructions and split it into two parts, attaching each part with a protein linkage to a different nanobody that recognizes the same marker. The two nanobodies will bind to their respective part of the marker, bringing the polymerase back together and allowing for it to express what is called a reporter protein. The reporter protein selected to be expressed, called LacZ, produces a colorful compound. This colorful compound indicates the amount of marker present. The more biomarkers present in a sample, the more nanobodies will bind and form whole polymerases that express more proteins and produce an intense color.
Modifying the machinery
Because this machine is so modular—a nanobody, linker, polymerase, and reporter protein—parts can be easily exchanged to perform different functions. This enables faster design of diagnostics with varying levels of sensitivity. Think of a multitool with a tip that can be exchanged depending on the task at hand.
Researchers demonstrate that the nanobody portion of the cellular tool can be easily exchanged with other nanobodies or even other classes of proteins called monobodies and DARPins that are also known to recognize and bind to biomarkers. When developing a biosensor for COVID-19, they identified that nanobodies failed to strongly detect the disease. So they attempted to use a DARPin as the recognizing portion of the tool instead. This dramatically improved the signal, demonstrating that simple changes can be made to expedite biosensor development.
To further establish the utility of swapping the recognition tool, the scientists attached a nanobody that recognizes the protein transthyretin (TTR)—an indicator of general nutritional health. This engineered tool detected TTR well below normal levels in human blood serum samples, establishing it as immediately relevant to clinical settings.
They also showed that the reporter protein can be exchanged. Previously, the polymerase would produce LacZ that creates a red color in the solution. Researchers swapped this for a green fluorescent protein (GFP), which allows the biosensor to be more easily designed and produced using established technology for the cell-free expression of proteins.
A deceptively simple path
While this path may seem simple, there is a lot of optimization that goes into designing the cellular tool. Pieces must be expertly planned so they fit together like a puzzle and carry out the desired reactions. One of the variables that can be modified to optimize the performance is the length of the linker protein between the polymerase and nanobody. Make this link too long and the polymerase fragment has too much free rein to roam. Too short and it might not be able to reach its other half.
The other optimization is deciding where nanobodies should bind on the biomarker. Having all the nanobodies compete for one spot does not create a functional tool. But the two binding spots cannot be so far away that the fragments will never form a complete polymerase.
RELATED: Predictive Power of Biomarkers in Pediatric ICU
Caveats to the tool’s performance
There is an important term to introduce when it comes to biosensing: limit of detection (LOD). This is a measurement of the lowest concentration that a biosensor can detect reliably. While the researchers were able to detect COVID-19 with their test, they could only measure it at a concentration greater than the LOD of other, commercially available rapid tests.
In general, the test does not have as good of performance as more common methods. However, it presents many advantages such as its affordability and ability to be easily modified for quick development of new sensors. While there is more work to be done in exploring what characteristics help guarantee success, investigating these variables allowed researchers to design a highly versatile, cellular tool that can be customized for different diseases.
RELATED: Nanoparticle Vaccine Created for Better Immune Response
Shelving it for the future
There is one final roadblock on the journey to getting this technology into the hands of those in hard-to-reach places. This liquid tool needs to be stable when stored at room temperature over time. To do this, researchers turned to lyophilization: a process of removing water through freeze-drying that allows for the preservation of easily perishable materials. When it is time for implementation, the tool can be re-hydrated and used with a human sample such as saliva or blood serum at the point-of-care.
Finally, the researchers have arrived at their destination with a diagnostic tool that can be handed to the people it is most useful to. But this work has only scraped the surface of the utility of this cellular tool. It may be expanded to target non-protein molecules such as fats or carbs and there is room to improve its reliability. So maybe this conclusion is just a pit stop on a larger path toward a new frontier of biosensors.
This study was published in the peer-reviewed journal Science Advances.
Reference
McSweeney, M. A., Patterson, A. T., Loeffler, K., Cuellar Lelo de Larrea, R., McNerney, M. P., Kane, R. S., & Styczynski, M. P. (2025). A modular cell-free protein biosensor platform using split T7 RNA polymerase. Science Advances, 11(8). https://doi.org/10.1126/sciadv.ado6280
Featured image shows the new cellular tool, which produces a simple color output based on the amount of protein detected in the sample. Credit: Candler Hobbs
About the Author
William Harley completed degrees in Biochemistry & Molecular Biology and Chemical Engineering from Oregon State University. While an engineer by profession, he enjoys creating music, trail running, and baking.



![Dichroa febrifuga, a medicinal herb that has been historically used to treat fever, is named for its active ingredient, febrifugine. By Keith Edkins (Own work) [CC BY-SA 3.0], via Wikimedia Commons](https://magazine.scienceconnected.org/wp-content/uploads/2016/05/Dichroa_febrifuga_Glasgow-e1463012377212.jpg)