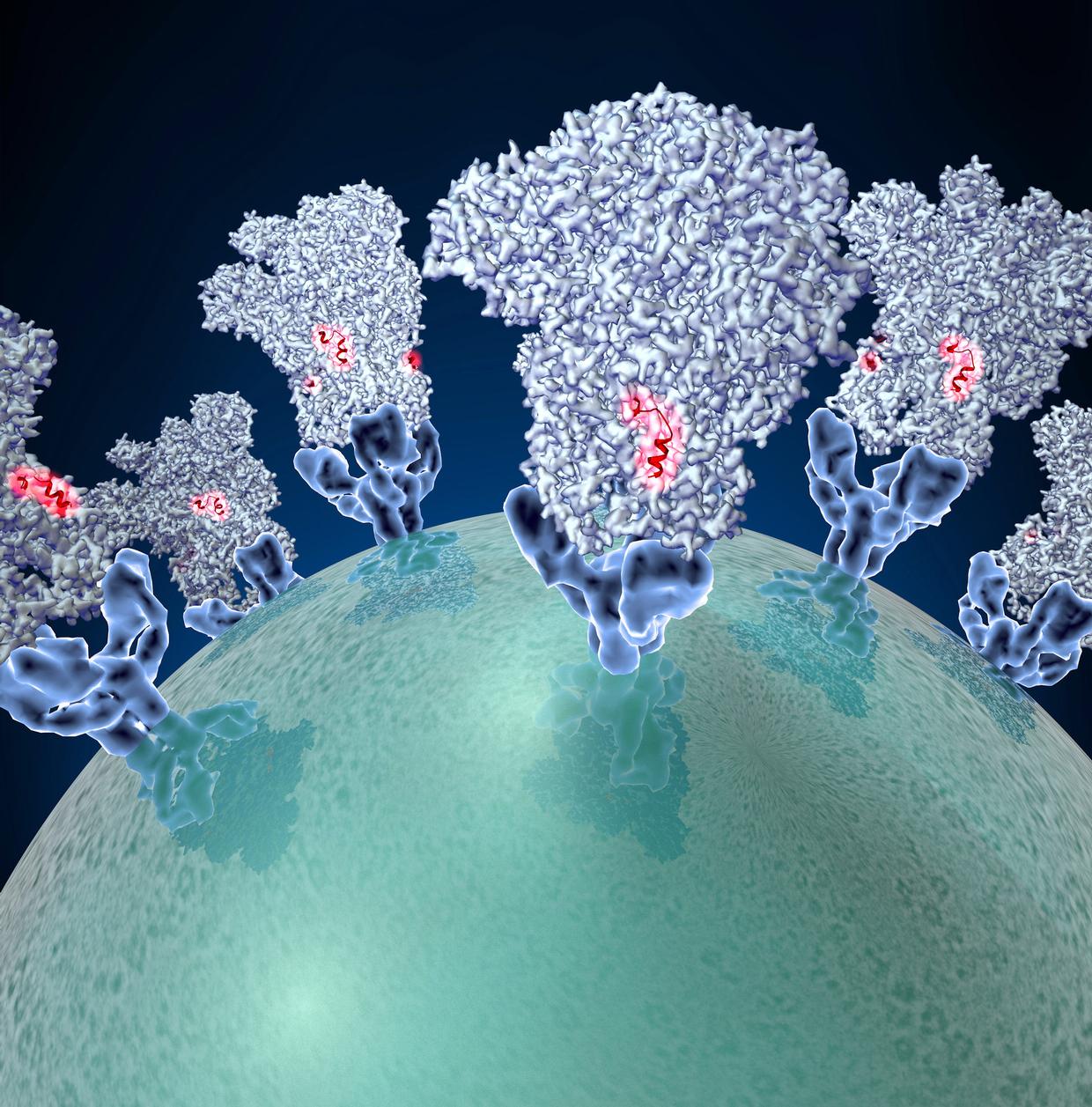
In a new study, a nanoparticle vaccine developed by MIT and Caltech could protect against SARS-CoV-2 variants and similar animal-borne coronaviruses.
By Antarjot Kaur
Researchers at MIT and Caltech have taken a groundbreaking step toward developing nanoparticle vaccines that protect against both current and future viruses. Using computational models, they have designed “mosaic nanoparticles” that can trigger strong immune responses. These vaccines aim to combat SARS-CoV-2 variants and other related viruses, potentially preventing future pandemics.
Why new vaccines are necessary
Since the COVID-19 pandemic began, the SARS-CoV-2 virus has evolved rapidly. Variants like Omicron have been able to evade immunity from earlier vaccines and infections. Existing vaccines, such as mRNA vaccines, can be updated to address new variants, but this process takes time and may not always keep up with viral changes. Additionally, there is a constant risk of new viruses emerging from animals and jumping to humans. The study researchers believe a universal vaccine is crucial to address these challenges.
RELATED: A Game Playing App May Have Just Helped Create COVID Vaccines for the Developing World
What are mosaic nanoparticles?
Mosaic nanoparticles are specially designed vaccine components that display parts of different viruses to the immune system. In this study, researchers attached receptor-binding domains (RBDs)—the part of the virus that allows it to infect human cells—from various SARS-like viruses onto nanoparticles. These nanoparticles were then used to create vaccines that can stimulate the immune system to target shared, conserved parts of these RBDs. This approach is designed to train the immune system to fight not just one virus but many related ones, including future variants.
RELATED: How Databases Signal Successful Immunotherapy
How the nanoparticle vaccines were made
To create the vaccine, the researchers used computer programs to pick the best RBDs of various viruses (including all variants of SARS-CoV-2) for inclusion. They carefully modified these to ensure they properly fold and do not induce weaker immune responses. The computer models also eliminated any significant overlapping of the RBDs to avoid confusing the immune system.
The researchers then employed a system called SpyCatcher-SpyTag to attach these RBDs to nanoparticles. Working in a “plug-and-play” manner, the scientists mix and match various RBDs on the same nanoparticle. This flexibility will enable them to alter the vaccine easily in case new variants or viruses emerge. The mosaic-7COM vaccine included RBDs from viruses known to infect humans and others hailing from animals to maximize the chances of broad protection.
Key findings from the study
The results showed that the new mosaic nanoparticles were highly effective:
- Stronger Immune Responses: Mice vaccinated with mosaic-7COM produced high levels of antibodies that could neutralize SARS-CoV-2 variants and other SARS-like viruses.
- Focus on Conserved Epitopes: The vaccines trained the immune system to target conserved parts of the virus that are less likely to mutate.
- Protection Against Diverse Viruses: Mosaic nanoparticles provided broad protection, including against heavily mutated Omicron variants and zoonotic viruses that have the potential to jump to humans.
- Effective in Vaccinated Mice: Even mice that had already received COVID-19 vaccines showed improved immune responses when given the mosaic-7COM vaccine. This effectiveness could indicate its potential as a booster.
Comparing mosaic vaccines to older approaches
Previous nanoparticle vaccines, such as the mosaic-8b, used a fixed set of virus parts. While effective, they did not adapt to the latest variants as efficiently. The mosaic-7COM vaccine, however, was designed using cutting-edge computational methods to include RBDs that target conserved regions across many viruses. This makes it more effective against a broader range of threats.
Also, conventional vaccine methods rely on “spike protein” structures that can change very quickly. Mosaic nanoparticles address this issue by targeting the RBD’s stable regions, which makes it more difficult for the virus to evade immune defenses.
RELATED: Measles Treatment Beyond Vaccines – A New Therapeutic Path
What’s next?
The mosaic nanoparticle vaccines have shown great promise in animal studies, but more research is needed before they can be used in humans. Scientists must conduct clinical trials to test their safety and effectiveness in humans. These vaccines could become critical tools in preventing future pandemics and protecting against unpredictable viral threats.
RELATED: “Flu Finders” Teaches What to Do When Sick
Why this matters
The development of mosaic nanoparticles represents a major step forward in vaccine technology. By preparing for the viruses of tomorrow, these innovations could help the world stay one step ahead of the next pandemic.
This study was published in the peer-reviewed journal Cell.
Featured image is “Coronavirus spike protein structure.” Credit: David Veesler, University of Washington, provided by NIH and licensed under CC BY-NC-SA 3.0. “The illustration shows a viral membrane decorated with spike glycoproteins; highlighted in red is a potential neutralization site, which is a protein sequence that might be used as a target for vaccines to combat viruses such as MERS-CoV and other coronaviruses.”
Reference
Wang, E., Cohen, A. A., Caldera, L. F., Keeffe, J. R., Rorick, A. V., Aida, Y. M., Gnanapragasam, P. N. P., Bjorkman, P. J., & Chakraborty, A. K. (2025). Designed mosaic nanoparticles enhance cross-reactive immune responses in mice. Cell. https://doi.org/10.1016/j.cell.2024.12.015

About the Author
Antarjot Kaur is a cognitive science undergraduate from India, passionate about healthcare policies, behavioral science, and neuroscience research focused on neurodegenerative memory disorders like Alzheimer’s, dementia, etc., and particularly involved in women’s health issues, education, and empowerment.