With a rising need for measles treatment worldwide, scientists have uncovered a new way to protect those who can’t be vaccinated for it.
By Tanja Eisemann
A team of scientists from the La Jolla Institute for Immunology and Columbia University discovered a novel treatment approach for measles virus infections. The scientists developed an antibody that interferes with the viral protein that enables the measles virus to infect human cells. They identified how the antibody changes the spatial arrangement of its viral target, providing insight into mechanisms of virus-cell fusions that are critical for the development of antiviral therapeutics.
The measles virus, a persistent threat
With an infectivity of almost 100 percent among unimmunized individuals, measles is one of the most contagious diseases we know of. Measles is a viral respiratory disease that typically manifests with fever, a sore throat and persistent cough, a runny nose, and a characteristic red, blotchy rash. However, especially in children, measles can cause severe complications including pneumonia and encephalitis leading to chronic disabilities or even death in 1 to 3 out of every 1,000 infected children. Luckily, the introduction of a measles vaccine in the 1960s and global immunization campaigns drastically reduced the infection rates, and with it, the number of fatal cases. The World Health Organization (WHO) reports that between 2000 and 2023, measles vaccination prevented estimated 60 million deaths worldwide. In 2000, measles was declared eliminated in the United States. Yet, over the past decade measles cases have been rising globally, with over 207,000 measles deaths recorded by the WHO, and 1,274 infections in the US in 2019.
Why vaccination rates are declining
How can this trend be explained, considering the availability of a safe and cost-effective vaccine? To reach protective immunization in a community, more than 95 percent of people need to be vaccinated or immune. This high vaccination rate is necessary because measles has a very high infection rate. This is indicated by the basic reproduction number R0, which for measles is around 18, meaning that each infected person can pass it on to 18 other unprotected people—for comparison: SARS-CoV-2 has an R0 value of 2. Several states have failed to reach this herd immunity in the past decade.
Numerous reasons contribute to why the vaccination rate dropped below 95 percent. This includes limited access to health care, especially during the COVID-19 pandemic lockdown years, increasing numbers of immunocompromised individuals that are not eligible to receive the vaccine, and misinformation eroding trust and confidence in vaccines. A report in 1998 claiming a causal link between measles vaccine and autism raised serious concerns among populations worldwide. Despite this report having been retracted by the publishing journal due to incorrect and fraudulent elements, and countless follow-up studies that could not associate autism and the vaccine, the myth of severe side effects has endured. Fueled by another wave of vaccine hesitancy sparked during the COVID-19 pandemic, the decrease of childhood vaccination rates resulted in globally increased numbers of measles cases in the 2020s, according to UNICEF.
RELATED: Vaccine Misinformation and Social Media
Antibody-based measles treatment shows promising results
Even if we can overcome vaccine hesitancy in the future, the measles vaccine comes with certain limitations. “The current vaccine cannot be taken by pregnant people or people with compromised immune systems,” says Dr. Dawid Zyla, scientist at the La Jolla Institute for Immunology (LJI). Zyla studies strategies to eradicate the measles virus in Dr. Erica Ollmann Saphire’s laboratory. The scientists worked on a novel measles treatment that can also be used by individuals who are not eligible for a vaccine-based measles protection.
In this study, co-led by Dr. Erica Ollmann Saphire and Dr. Matteo Porotto, Professor of Viral Molecular Pathogenesis at Columbia University, a team of scientists investigated the function of an antibody they had previously discovered, mAb77. Using cell culture and rat models, they found that mAb77 efficiently blocks infection by the measles virus, suggesting a protective effect for humans too. This promising finding raises hope for future clinical applications, though further tests are needed to determine if the antibody can also stop the virus from spreading once infection has occurred in human patients.
RELATED: Can viruses be used as medicine? Read more in Giant Virus Evolution Key to New Medical Nanotechnology
Uncovering the mechanism behind mAb77
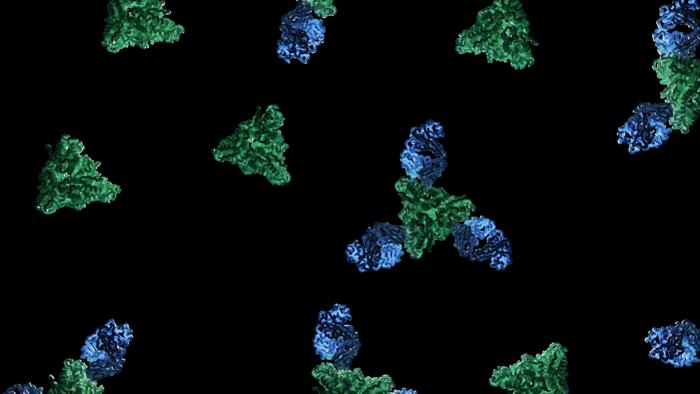
In addition to these exciting findings, the scientists also considered the underlying mechanisms. The mAb77 antibody prevents infection by blocking the fusion of the virus with its target cells. This process is a critical step during viral infection. A specific protein on the virus envelope, called an attachment protein, binds to a surface protein, the receptor, on the target cell. This activates a second viral protein, called fusion or F protein, that mediates the fusion of the viral and the host cell granting the virus access to its target cell.
“Understanding the process of viral entry, the first step in the viral pathogenesis, is basis for development of potent antiviral treatments. If we know how something works, we might figure out how to stop it,” says Zyla, first-author of the study.
The team of structural biologists investigated what happens to the conformation and function of the F protein once the antibody binds. For this analysis, Zyla applied cryo-electron microscopy, a sophisticated method to image biomolecules with high precision. Invented as an offshoot of electron microscopy, this Nobel-Prize-winning technique fires beams of electrons at frozen proteins in ice. The electrons that interacted with the protein sample then create a high-resolution image on an electron detector.
The scientists first produced the viral proteins in sufficient numbers and stability for their structural investigations. They then combined the viral proteins and the mAb77 antibody. They analyzed the frozen mixture by cryo-electron microscopy, capturing snapshots of the antibody’s effect throughout the fusion process. When the researchers added the antibody, they found that the F protein could still start to change its shape but was then arrested in a transitioning state before completing the fusion. The data provided for the first time high-resolution insight into the intermediate steps of the measles fusion process.
Implications for future measles treatments
In a commentary article, Dr. Hector Aguilar, an outside expert in virology at Cornell University, praises the study. He sees its advancements in the analysis of fusion dynamics as crucial for designing antiviral drugs. In particular, he emphasizes the value of these findings for the development of therapeutics against other paramyxoviruses. Nipah and Hendra viruses are closely related to measles, and they all have very similar protein structures. The new mechanistic understanding of the membrane fusion cascade may help to develop novel therapies for these viruses, for which there is currently neither a therapy nor a vaccination.
The decades after introducing the measles vaccine and the severe drop in infection and death rates have proven the great effectiveness and safety of the vaccine. Thus, the researchers from LJI and Columbia University do not intend to replace the vaccine with their antibody-based measles treatment. “The currently used vaccine is a success story in virology and vaccine development. It is safe, inexpensive, and, with adequate coverage, could have eradicated measles long ago,” Zyla says. He also explains though, that due to advancements in other medical fields, such as cancer therapy and organ transplantation, the population of individuals who are immunocompromised and cannot receive the current vaccine is increasing. “Furthermore, small children under one year of age, who cannot yet receive the vaccine, are also potentially exposed to measles. We hope that our study will contribute to the development of new antiviral strategies, such as antibody cocktails, that can help people exposed to this virus.”
This study was published in the peer-reviewed journal Science.
References
Aguilar, H. C. (2024). Antibody inhibition of measles virus entry. Science, 384(6703), 1406–1407. https://doi.org/10.1126/science.adq3348
Zyla, D. S., Della Marca, R., Niemeyer, G., Zipursky, G., Stearns, K., Leedale, C., Sobolik, E. B., Callaway, H. M., Hariharan, C., Peng, W., Parekh, D., Marcink, T. C., Diaz Avalos, R., Horvat, B., Mathieu, C., Snijder, J., Greninger, A. L., Hastie, K. M., Niewiesk, S., Moscona, A., Porotto, M., & Ollmann Saphire, E. (2024). A neutralizing antibody prevents postfusion transition of measles virus fusion protein. Science, 384(6703), eadm8693. https://doi.org/10.1126/science.adm8693
Feature image: As captured by structural biologist Dawid Zyla in this study, image shows the structure of a new neutralizing antibody (blue) binding to the measles fusion glycoprotein (green) to elicit a neutralizing mechanism. Credit: Dawid Zyla, LJI.

About the Author
Tanja Eisemann earned her PhD at the German Cancer Research Center and currently studies brain tumor immunology at the Sanford Burnham Prebys Medical Discovery Institute. She lives in southern California with her husband and son, enjoying the beautiful weather and nature when she’s not eagerly working at the laboratory bench. Follow her at: https://www.linkedin.com/in/tanja-eisemann/.