Find out why the Periodic Table of Elements is systematically brilliant. Also, get two free periodic tables that you can download and print for your classroom or dining room wall.
Series: The Periodic Table and Sorting Elements – Part 3
Sorting Hat: Houses
The Periodic Table is brilliant for providing information about each element systematically. The atomic number tells us how many protons (the positively charged component of the nucleus) and how many electrons (negatively charged particles outside the nucleus) each element has. Looking at the atomic weight, we can quickly understand how many neutrons may be in the nucleus alongside the protons. With fractional atomic weights, we know there are multiple isotopes of the atom.
Rows are associated with increased numbers of protons, neutrons, and electrons. Generally, the further down the Periodic Table one goes, the more likely it is that an element is radioactive.
The organization of the Periodic Table in Rows and Columns is not haphazard. For Harry Potter fans, think of it as an algorithmic version of a sorting hat. Hmmm, is this element a Ravenclaw or Hufflepuff?
Versions of the Periodic Table
While the information about each element in the Periodic Table is the same, different formats have been used to organize the Periodic Table. The Periodic Table has also evolved. Let us take a brief look at both the history and the most common versions in use today.
John Newlands organized elements based on weights. He observed there were similar properties every eight elements, so he organized his table with eight columns. He left no gaps in his table.
In 1863 Dmitri Mendeleev drafted the first of his 60 versions of the Periodic Table. He used atomic weights to sort the elements; however, he reordered them based on observed properties if they did not seem to be in the right spots. This was accurate, although speculative. Mendeleev trusted experimental evidence of chemical reactions more than the measured weights. He was correct because many elements were difficult to isolate in the samples at the time. Frequently incorrect weights were reported and later revised when purer samples could be analyzed.
Mendeleev left gaps where there was no known element matching the anticipated weight and properties. Mendeleev’s gap for Gallium (Eka-Aluminium) is a well-known example. By 1871 Mendeleev had a Periodic Table in eight groups related to oxidation states. This 8-column format was used for decades, even after other formats were developed.
Skipping ahead to 1913, Anton van den Broek, proposed the nuclear charge determined the placement in the Periodic Table. In 1914 Johannes Rydberg determined a relationship in the atomic numbers of noble gases. This led to the octet rule and valence bond theories. This was further adapted into the Bohr model. Combined with Pauli’s exclusion principle a quantum rule for filling electron shells was determined. Finally, Glenn Seaborg proposed the f-series (Actinide Series) based on his research on Americium and Curium.
“I believe that the chief difference is that you [Glenn Seaborg]are using the periodic table to express the probable configuration of the electron shells, while I and a few other chemists are primarily concerned with the representation of the chemical character of the elements.” – F.A.Paneth to G.T.Seaborg, 14 July 1950, Box 342, Glenn Theodore Seaborg Papers, Manuscript Division, Library of Congress, Washington, D.C.; c.f. Doctoral Dissertation, Creating a Symbol of Science: The Development of a Standard Periodic Table of the Elements Ann Robinson, p. 247
The discovery of many more elements than originally in Mendeleev’s Table, increased understanding of the nucleus (protons and neutrons), as well as electron orbitals, led to the modern Periodic Table.
There were several forms of the Periodic Table used in textbooks in the 1950s. Some were 8-column, 18-column, and 32-column tables. Others were arrangements referred to as “rocket ships,” based on Niels Bohr’s early Periodic Tables.
Today’s most used version of the Periodic Table evolved from Horace G. Deming’s 1923 General Chemistry textbook. It contains 18 columns and is itself derived from Alfred Wagner’s 1905 18-column “block” layout reflecting the s-, d-, and p-blocks (sub-shells) [1]. The long version with the f-block “inline” has 32 columns.
Deming’s table achieved a breakthrough in 1928 because the publisher distributed U.S. letter-size printouts as part of a promotional campaign. These continued to be provided with new editions for several decades. The wide distribution of these materials in Western countries and the practical format led to this 18-column form becoming the most popular version.
The 18-column version of the Periodic Table is not superior to other versions. Indeed the 32-column format has multiple advantages; however, it has a significant disadvantage: layout space. The 18-column version is more compact with a favorable aspect ratio allowing it to fit easily on textbook pages or handouts. The 32-column format requires foldouts or much longer printed charts. Even in our digital world, aspect ratios on web pages and computer monitors or smartphones typically favor the 18-column design.
In summary, there is no official version of the Periodic Table approved by the International Union of Pure and Applied Chemistry (IUPAC) or other bodies. A widely used version of the Periodic Table in 18-column format has been used since the 1950s and can trace its usage back to Deming’s General Chemistry (1923) and even before that to Alfred Wegner’s 1905 “block” layout.
Today’s primary use of the Periodic Table is education. There have been adaptations to reflect various aspects of the elements better: sometimes these focused on chemical characteristics, and sometimes there was focus on physical aspects.
Improving the Periodic Table for Students
In my opinion, as brilliant as the Periodic Table already is, it can be improved further using our current understanding of atoms. A slight modification of the block arrangement underlying the “Deming” Periodic Table allows for a more consistent representation in terms of sub-shells and the associated properties of the elements.
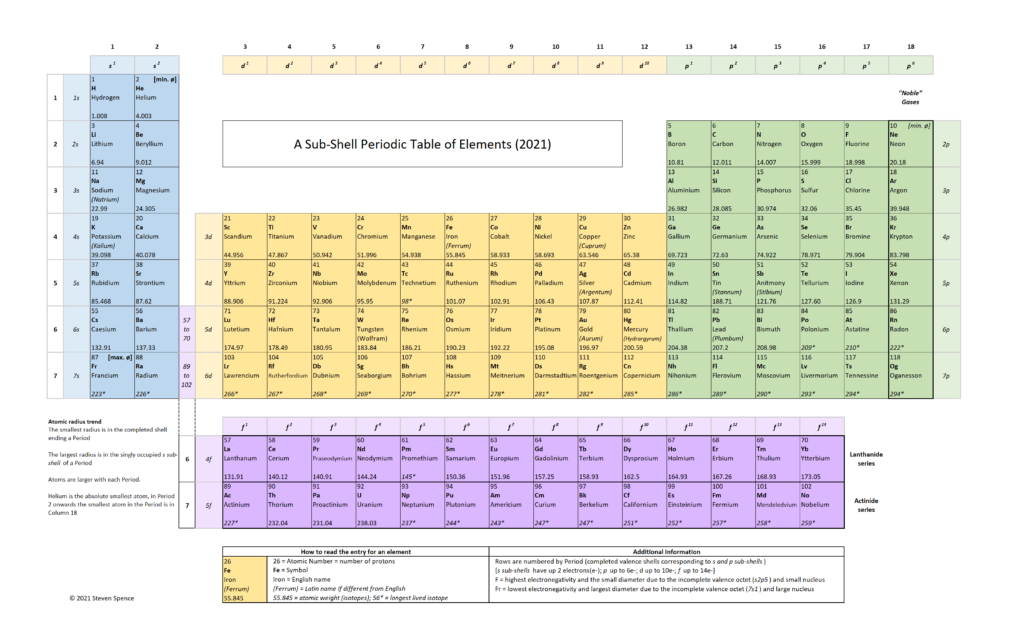
Tweak 1
By convention, Helium [He; Element 2] is in the rightmost column (18) of the standard Periodic Table depiction. Why? Because it completes a “Period,” which is the same as a row. Conventionally, all elements with complete Period end in Column 18 (aka Group 18). This is also a convention related to the “octet rule” and “noble” gases. However, it creates a sub-shell inconsistency in the tabular representation.
- A row (Period) on the Periodic Table reflects a series of electron sub-shells. A Period is closed out when all the sub-shells in the row are filled with electrons. The first Period (row) consists only of the s sub-shell, which is closed when there are two electrons. Splitting the representation of the s sub-shell into columns 1 and 18 for Period 1 while all other s sub-shell Periods are in columns 1 and 2 is visually misleading.
- When all the valence sub-shells (s- and p-) in a Period are filled, the last element in that Period tends to remain unbonded with other atoms, which is why all the “noble” gases are in Column 18. Helium behaves chemically like a “noble” gas because it only has one sub-shell. This is easy to explain to students as a special case, i.e., Period 1 does not have a p- sub-shell. Students benefit from Helium being in its natural position with other s2 elements.
Tweak 2
In some versions of the Periodic Table, I see the spaces in Row 6 and Row 7 in column 3 (under 39 Yttrium) referring to the Lanthanide and Actinide series, which are generally depicted in rows below the main Periodic Table in the 18-column version. In the 32-column version, this is not necessary as the 14-columns required for the f sub-shell can be depicted “inline”.
In these 18-column versions of the Periodic Table, the series includes 15 elements in the Lanthanide (row 6) and Actinide (row 7) series. While this is not a significant issue because very few students will ever need to work with these elements, a different depiction would be more consistent with the sub-shells. The f sub-shell contains 14 electrons and not 15, as a student might incorrectly conclude from the Lanthanide and Actinide series representation.
I suggest adding a reference column, which allows the f sub-shell to break out below the main Periodic Table cleanly. The Lanthanide Series’s revised form consists of 14 elements in the f sub-shell ranging from Lanthanum [Element 57] to Ytterbium [Element 70]. Lutetium [Element 71] is in Column 3 as a d-shell element.
Similarly, the Actinide Series consists of 14 elements in the f sub-shell ranging from Actinium [Element 89] to Nobelium [Element 102]. Lawrencium [Element 103} is in Column 3 as a d-shell element.
Due to similar energy levels in the f and d sub-shells, there are anomalies in the Lanthanide and Actinide series filling order. By the time Element 71 and Element 103 are reached, the f sub-shell is complete. The value of the general principle illustrated outweighs the exceptions in the electron filling order when classifying the elements.
Tweak 3
I choose to leave out color-coding and thick lines showing Alkalines, Transition Metals, etc. Many Periodic Table representations have a high information density due to such color coding, but it can also be confusing.
I feel simpler is often better. As with any tool, it is crucial to understand its purpose. My tweaked version of the Periodic Table focuses on electron sub-shells as the key to unlocking chemical attributes. Recognizing an element as an Alkaline metal or a “Noble” gas becomes a derived function of the valence electron configuration in sub-shells rather than memorized by color-coding.
There are valid and practical reasons for today’s commonly used Periodic Table representations. At the same time, I think there is also room for a more consistent sub-shell depiction to convey the underlying principles to students better. Once fundamentals are clear, it is easier for students to grasp how and why the chemical properties of elements vary.
Conclusion
The Periodic Table is a powerful learning aid. It has evolved to reflect and organize data about elements systematically. In the past, gaps in the Periodic Table pointed to elements that were yet to be discovered. The Periodic Table also enabled scientists to make predictions about the characteristics of the new elements before their discovery.
Today’s most used form of the Periodic Table is an 18-column version widely adopted in the 1950s and 1960s. It derives from 1905 and 1923 “block” representations of the Periodic Table. A block representation reflects the underlying s, f, d, and p sub-shells. The currently used version has a few minor representational inconsistencies, which may cloud a student’s understanding of the sub-shells and related chemistry. A suggested revision accompanies this post for the reader’s consideration.
Speculative Bonus
Building upon the more consistent representation in the revised version, I have also proposed a hypothetical Periodic Table to incorporate Period-8 elements. It assumes the 5g sub-shell will follow the “Aufbau” Principle in how sub-shells fill. We know there are some exceptions to this rule for the Lanthanide and Actinide series. I fully expect exceptions in the 5g sub-shell, which I label the “Super Actinide” series as an easy-to-remember shorthand.
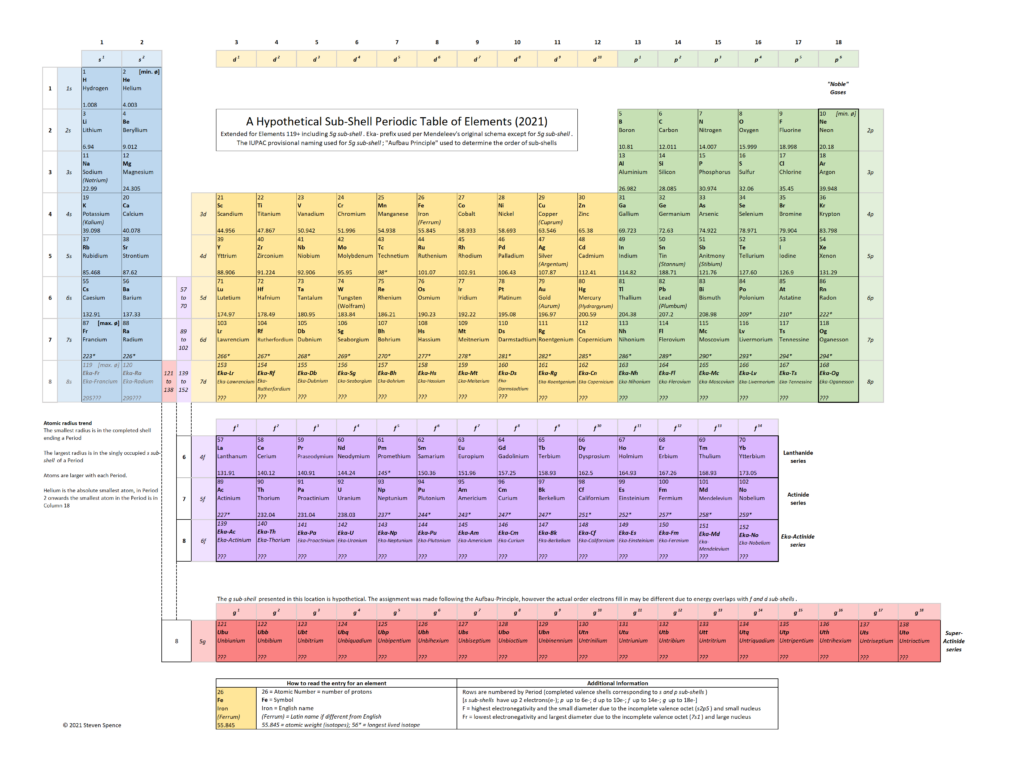
As physicists continue to make progress in synthesizing elements, the Period 8 series will move from hypothetical to experimentally relevant. The characteristics, stability (island of stability?), and the electron orbital filling order of these new elements will tell us empirically what the correct placement of the 5g sub-shell should be. I am eager to see the experimental results.
Read the Series
The Difference Between Chemical and Nuclear Reactions
Pull up a chair and sit down to the Periodic Table and Sorting Elements – Part 1: The Difference Between Chemical and Nuclear Reactions.
Chemical Reactions: Periodic Genius
Chemical reactions are essential. They put oxygen in our blood, salt in our food, and batteries in our phones. Find out why the periodical table is so brilliant.
Find out why the Periodic Table of Elements is systematically brilliant. Also, get two free periodic tables that you can download and print for your classroom or dining room wall.
Endnotes
If you wonder (as I did) why the sub-shells are labeled s, p, d, and f, it is due to an obsolete method of categorizing spectral lines as “sharp,” “principal,” “diffuse,” or “fundamental.” Today sub-shells beyond f will follow the alphabet. The next sub-shell will be the g sub-shell with 18 electrons, then the h sub-shell with 22 electrons, the i sub-shell with 26 electrons, “j” will be skipped due to similarity with the letter “i”; the k sub-shell with 30 electrons, and so on. Synthesis of elements may not ever progress far enough to need a sub-shell beyond the g sub-shell due to the energies involved. Furthermore, some atomic nuclei and electron orbits calculations indicate that a limit exists at theoretical Element 173. Search for Dirac equations and the Bohr model to learn more.
- An excellent single source of information on the history and evolution of the Periodic Table is: Doctoral Dissertation, Creating a Symbol of Science: The Development of a Standard Periodic Table of the Elements, Ann Robinson. An online version is available at: https://core.ac.uk/download/pdf/220129765.pdf
- Here is my suggestion for the sub-shell representation of the Periodic Table in PDF format
- Here is my hypothetical extension of the suggested Periodic Table for the Period 8 Elements including the 5g sub-shell in PDF format.