Glucose monitoring patches developed to be more flexible and comfortable, offering better diabetes treatment.
By Adelaide Levenson
As of 2021, over 10 percent of the global population is living with diabetes. This disease leads to elevated blood glucose levels, which can require around-the-clock monitoring. Current methods for tracking blood glucose levels can be uncomfortable or costly. With new technologies emerging, such as microneedle sensors, there is room for improvement within this field. One team of scientists at Imperial College London set out to develop a flexible, less invasive, wearable microneedle patch for glucose detection.
Diabetes and glucose monitoring methods
Diabetes can be separated into a few different categories. The most common diagnoses are Type 1, Type 2, and gestational diabetes. Type 1 diabetes causes the immune system to attack the cells that produce insulin in the pancreas. With Type 2 diabetes, the pancreas either makes less insulin than it needs or the body develops a resistance to insulin. Without sufficient amounts of insulin, blood glucose (or blood sugar) begins to rise. Diabetes that occurs during pregnancy, and typically goes away afterwards, is referred to as gestational diabetes. Regardless of the specific type of diabetes, glucose monitoring plays a crucial role in management. The results can indicate when insulin might need to be administered.
For individuals affected by diabetes, it is beneficial to use a device for continuous glucose monitoring (CGM). These devices keep track of glucose levels continuously throughout the day. Rather than relying on blood for glucose monitoring, CGM devices can use interstitial fluid (ISF) instead. ISF is a fluid that surrounds cells and has a similar composition to plasma, which is a major component of blood. The similarities between plasma and ISF make ISF a reliable alternative for glucose detection.
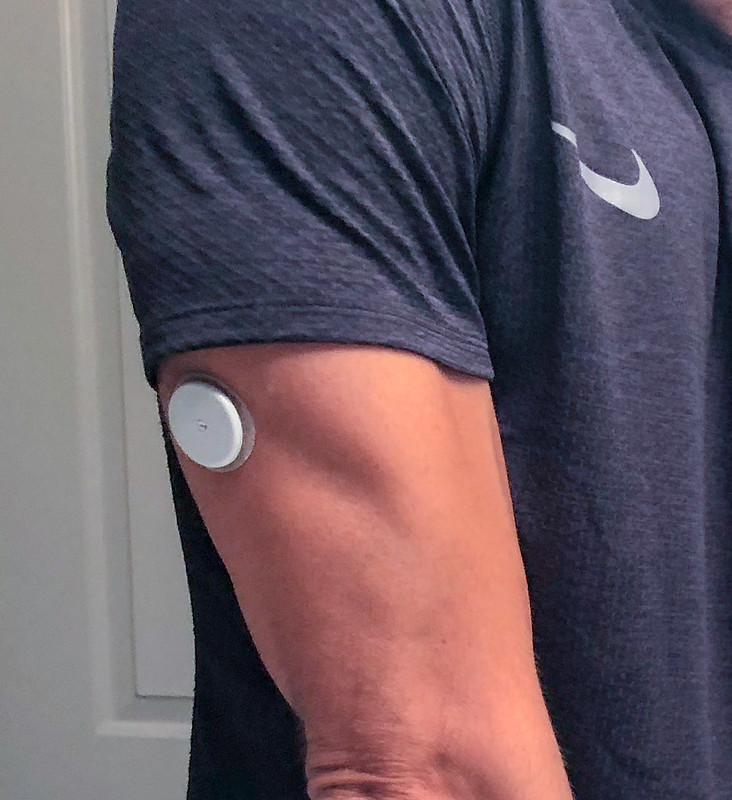
Photo courtesy of “2018.09.11 Continuous Glucose Monitoring, Washington, DC USA 1287” by tedeytan, licensed under CC BY-SA 2.0.
A drawback of using the current CGM devices is the requirement of long needles for sampling. These needles can range from around 5 to 11 mm—roughly the width of a pencil to the length of a bee. Alternatively, integrating microneedles into CGM devices would make them more comfortable. Unlike the needles on other devices, microneedles are shorter and don’t reach blood vessels, therefore causing less pain.
A new flexible and wearable patch
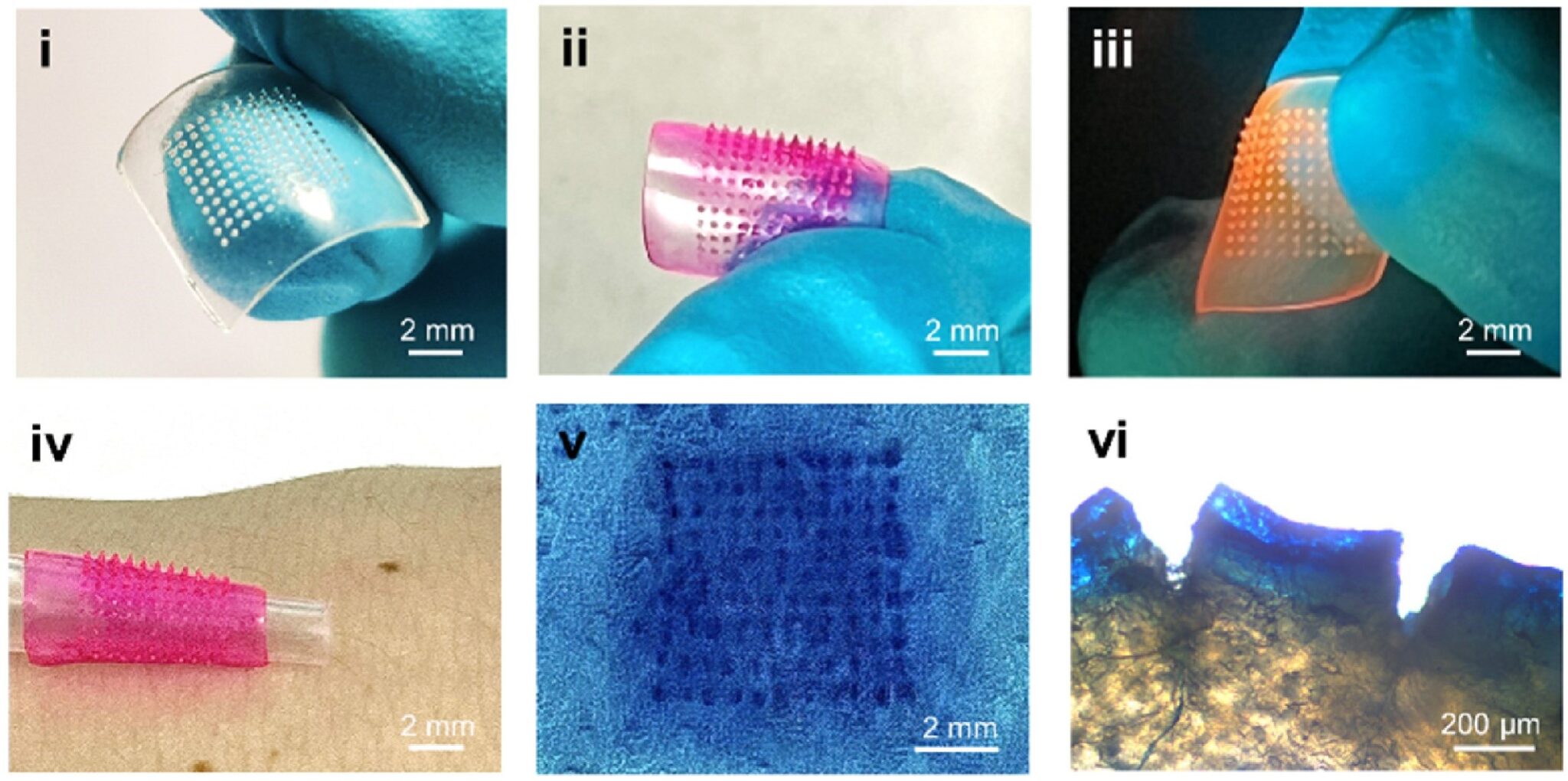
In addition to microneedle devices, hydrogels (water-based soft materials) have also had growing success and popularity in the last few years. The research team at Imperial College London detailed a glucose monitoring system that combines both of these techniques (microneedles and hydrogels) with Förster resonance energy transfer (FRET). Despite the long name, FRET produces easy-to-read signals; the output is color-based. FRET is a distance-dependent process that occurs when a donor molecule transfers energy to an acceptor molecule. In terms of FRET, a donor is typically a fluorophore, a compound that can absorb light and then emit the light as a different wavelength. When a FRET donor is in close range with an acceptor, instead of the donor emitting light, the energy is transferred to the acceptor. When this process occurs (donor and acceptor in close proximity), the color output is red. If the donor and acceptor are farther apart, there is less energy transfer and the readout is green. The team of researchers saw the benefit of combining these features (hydrogels, microneedles, and FRET). As a result, they were able to produce a flexible and easy-to-modify glucose sensor.
The first step in producing this sensor was to create the hydrogel-based microneedle patch. They achieved this by forming their sample in a microneedle mold. The patch, equipped with microneedles, was then coated in another layer. The second layer contained a FRET donor and acceptor, as well as a glucose receptor. The glucose receptor is used to monitor the concentration of glucose in ISF. If glucose is present, it will bind with the glucose receptor.
Once produced, the hydrogel in the patch is in its “shrunken” state, keeping the FRET acceptor and donor in close proximity. This gives a red readout. When glucose is present, it binds to the patch, causing the hydrogel to swell. As the swelling occurs, the FRET donor and acceptor get farther apart; shifting the readout to green. The more glucose that is present, the greener the readout becomes. With little to no glucose present, the patch stays red. Different glucose levels produce a range of colors between green and red, allowing for easy glucose concentration monitoring.
RELATED: Blue and Red Light: Exploring the Antimicrobial Potential of LEDs
Testing out the glucose monitoring patch
To test durability, researchers applied the patch to porcine (pig) skin and found that the patch remained intact upon removal. The microneedles penetrated about 266 µm into the porcine skin, which is 2.5 times the diameter of the average human hair. This study confirmed that the patch is flexible and robust.
Lastly, with the optimized patch, the researchers tested its performance in monitoring glucose changes throughout the typical day of a diabetic. To do this, they placed the patch on porcine skin and employed different concentrations of glucose to simulate meals. For “breakfast,” “lunch,” and “dinner,” they introduced glucose levels to mimic hypoglycemia, euglycemia, and hyperglycemia. With every change in glucose levels, the sensor responded with a different colored FRET output. The researchers monitored the patch over 10 hours and found the average response time to be 38 minutes. They claim that the sensor demonstrated good response to glucose concentrations within the clinically relevant range.
RELATED: Sweet Solution: Taste Protein Guides Drug Design
Looking toward further research
Although this glucose-sensing patch is an interesting addition to the field, there is still a lot of work to be done before it’s on the market. The authors mention that some of their next goals are in vivo studies and clinical trials. In addition to this, a few more issues could be addressed: How often does the patch need to be replaced? How is the patch secured to the skin? Additionally, as 38 minutes seems like a long response time, is there a way to produce a quicker readout?
New technologies are constantly emerging, offering innovative solutions to real-world problems. This microneedle patch for continuous glucose monitoring represents an exciting step forward in diabetes management. It has the potential to provide a minimally invasive, wearable, and real-time sensing approach. While further development is needed, devices like these could significantly improve disease management, making it an easier and smarter process.
This study was published in the peer-reviewed journal Biosensors and Bioelectronics.
Reference
Hu, Y., Pan, Z., De Bock, M., Tan, T. X., Wang, Y., Shi, Y., Yan, N., Yetisen, A. K. (2024). A wearable microneedle patch incorporating reversible FRET-based hydrogel sensors for continuous glucose monitoring. Biosensors and Bioelectronics, 262, 116542. https://doi.org/10.1016/j.bios.2024.116542
Learn more at:
American Diabetes Association. Diabetes Research, Education, and Advocacy. https://diabetes.org/
Diabetes. Cleveland Clinic. (2023, February 17). https://my.clevelandclinic.org/health/diseases/7104-diabetes
The information contained in this article is for informational purposes only and is not intended as health or medical advice. Always consult a physician or other qualified health provider regarding any questions you may have about a medical condition or health objectives.
About the Author
Adelaide Levenson is a chemist and baker with a passion for science communication. She recently received her MSc in chemistry with a specific focus on polymers, and currently resides in Germany.